Archaebiotics
see the origin of the concept archaebiotics here
Next generation probiotics - Prophylactic pharmabiotics in order to prevent diseases like atherosclerosis or trimethylaminuria?
Intestinal microbiota and food, an union that can turn morbid...
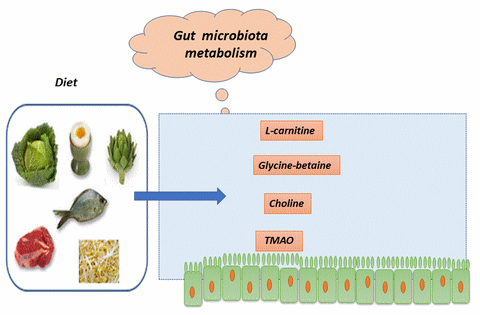
Since the works of Stan Hazen (Cleveland, Ohio, 2011), components of our diet are known to give rise to a compound found in the blood and very deleterious, under the action of certain bacteria of the intestinal microbiota.
These compounds / nutrients are more particularly phosphatidylcholine (or lecithin) / choline, L-carnitine, TMAO (or trimethylamine oxide) and glycyl-betaine.
Most of these compounds are also necessary / mandatory in certain quantities. They are then absorbed and used by our body.
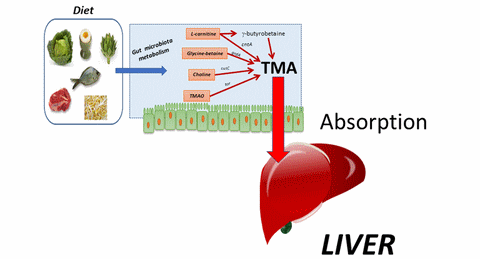
However, some of them can be used by some gut microbiota bacteria, most of them still unknown in 2016-2017. Some of these bacteria have enzymes that can metabolize (anaerobically) these compounds.
This results in the formation of trimethylamine or TMA in the intestine. This TMA is absorbed by the intestinal epithelium and will join the liver by the portal vein.
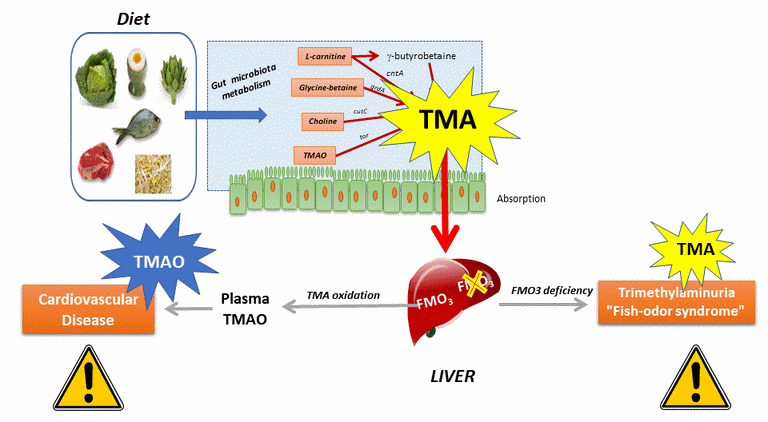
In the liver, this molecule can have 2 different fates depending on the activity of a liver enzyme, Flavin Monoxygenase 3 or FMO3.
- For the majority of people, this enzyme is functional (left arrow on the figure). TMA of intestinal origin then undergoes oxidation and leads to the formation of trimethylamine oxide (TMAO), a molecule that Stan Hazen and collaborators have revealed to be an important cardiovascular risk factor, which actively participates in various mechanisms involved in atherosclerosis. See here for more details.
- For some people, the liver enzyme FMO3 is not functional or absent, for genetic reasons (rare, autosomal recessive disease, with a carrier heterozygote rate in the UK of 0.5 to 1% (see here). In that case, TMA will diffuse in different body fluids. However, this molecule is very volatile and especially ... very smelly: it is indeed the one which gives its characteristic smell to rotten fishes, to which we are extremely sensitive. People with this pathology therefore present a trimethylaminuria (presence of trimethylamine TMA, in the urine) where it is detected to diagnose this pathology. Also called "Fish-odor syndrome", this pathology is psycho-socially very disabling.
Which way to prevent these diseases?
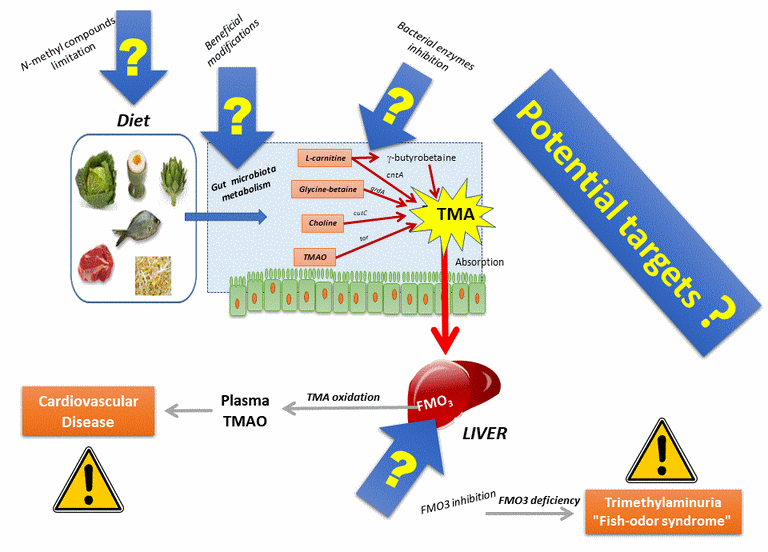
Another potential prophylactic way, the use of archaea?
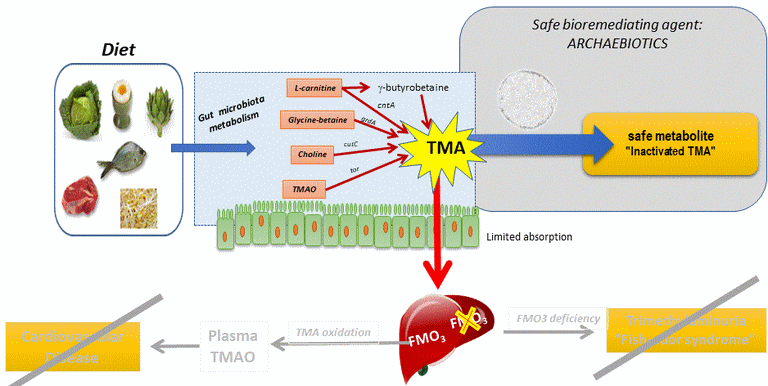
Archaebiotics
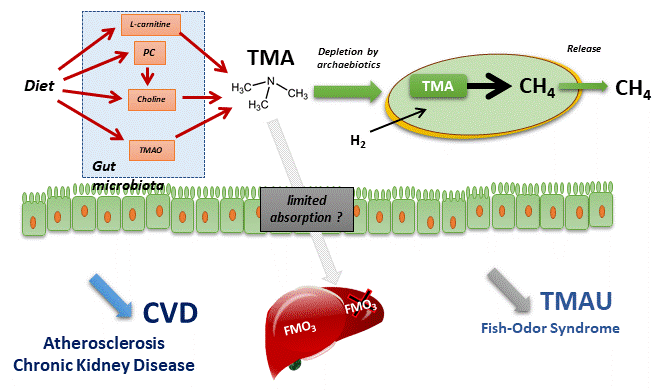
Methanosarcinales (absent from human intestinal microbiomes) and Methanomassiliicoccales (and not all).
This approach is described (originally) in the scientific article available here , and in a more simple way in a book chapter here.
Titre du paragraphe
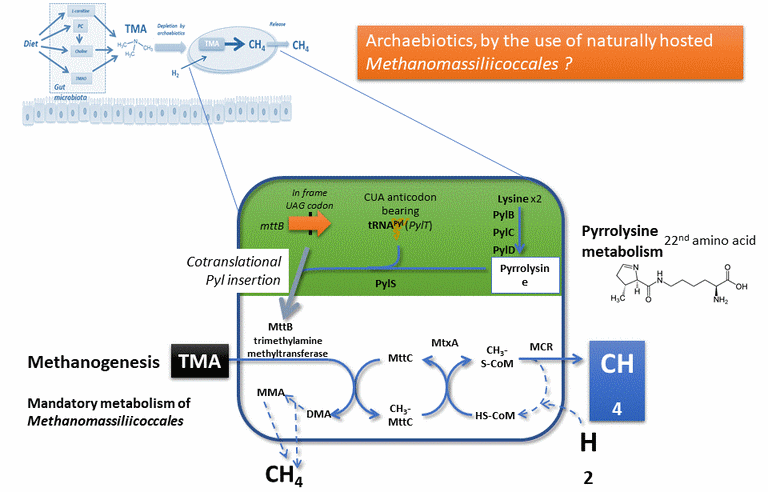
For a more advanced description of the Pyl system (figure representing a 3D modeling of the structure of the amber codon suppressor tRNA-Pyl, in Methanomethylophilus alvus), you can consult some of our scientific articles, especially this one. Also, see the dedicated page on this site.
A conceivable approach?
Some of the important benefits of this approach include:
- that there are no known pathogens in archaea (or for humans, animals or even plants!), which makes the idea promising, knowing that some Methanomassiliicoccales are naturally present in the human intestinal microbiota
- that this metabolism of methanogenesis is an obligatory metabolism for these species, which can only be realized with substrates other than TMA: this allows to consider an efficiency in the conversion of thisTMA.
- Important technological developments will have to be made before reaching this end, especially in the capacity to produce and keep active these strains supposedly extremely sensitive to oxygen.